Introduction: Follicular lymphoma (FL) accounts for about 35% of all non-Hodgkin lymphomas and 70% of indolent lymphomas in the United States. Despite relatively high 1L remission rates, a clinically high-risk subgroup experiences early disease progression within 24 months of 1L initiation (POD24). As the understanding of this population evolves, a treatment paradigm is yet to be established. This study aimed to describe the characteristics of patients who experienced POD24 to 1L therapy and their outcomes following receipt of select 2L treatment sequences using a contemporary real-world (rw) dataset.
Methods: Pts meeting the study criteria were identified in the COTA rw database. Eligible pts were aged ≥ 18 years at diagnosis (dx) with grade I-IIIA FL and had no documented grade IIIB or transformation anytime from dx until index date. Pts were treated with 1L CD20+chemotherapy (CHOP/CVP or Benda) and initiated 2L therapy with one of the following treatment categories between January 1, 2010 and December 31, 2019: Benda-CD20, CHOP/CVP-CD20, chemosalvage (e.g., R-ICE), or other novel therapy (e.g., lenalidomide-CD20). Pts were excluded if they were not treated with the specified 1L or 2L therapies, had missing or imprecise key study dates, multiple concurrent primary malignancies at dx, evidence of histologic transformation prior to 2L,1L or 2L clinical trial participation, or documentation of 1L maintenance therapy that did not contain CD20. Characteristics, treatment patterns, and outcomes were analyzed among pts who experienced POD24, defined as documented progression/relapse event within 24 months of 1L initiation. The index date was the date of 2L therapy initiation, and the observation period was defined as the duration of time from index date to the date of death or last visit date (if date of death was not available). Characteristics and treatment patterns were summarized as appropriate using means, medians, and/or counts and percentages. The following rw time to event outcomes were evaluated using the Kaplan-Meier method: time to next treatment (rwTTNT), progression-free survival (rwPFS), and overall survival (rwOS). Rw complete response rate (rwCRR) was calculated as the proportion of pts who achieved a complete response (CR) as the best response to 2L among all POD24 pts.
Results: The study included 149 pts who experienced POD24 and met the remaining study criteria. An additional 170 pts (53.3%) qualified for the study but did not experience POD24. The median age at dx for POD24 pts was 61 years. This population was mostly male (57.0%), White (87.9%), and treated in the community practice setting (86.6%). More than 85% of pts had low grade FL at their last assessment at index. Following 1L therapy, 47.0%, 1.3%, 39.6%, and 0.7% of pts had a documented CR, near CR, partial response, or stable disease, respectively. About 11% of pts had progression as their best documented response to 1L.
The most common 2L treatment category was Benda+CD20 (47.0%), followed by CHOP/CVP-CD20 (25.5%), chemosalvage (17.4%), and other novel therapies (10.0%). The CD20 received most commonly in 2L was rituximab (87.2%). Of 68 pts who received 3L, 11 (16.2%) received stem cell transplant.
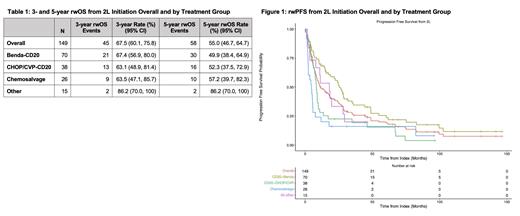
Median rwOS from 2L initiation overall was 64.8 months (95% CI: 54.0, 99.6). For pts who received Benda-CD20, CHOP/CVP-CD20, chemosalvage, and other novel therapies in 2L, 5-year rwOS rate from 2L initiation was 49.9%, 52.3%, 57.2%, and 86.2%, respectively. (Table 1) Median rwPFS from 2L initiation overall was 11.3 months, and pts who received Benda-CD20 or other novel therapies in 2L experienced median rwPFS of 25.6 and 18.0 months, respectively, while pts who received CHOP/CVP-CD20 or chemosalvage in 2L experienced median rwPFS of 8.3 and 4.6 months, respectively. (Figure 1) A similar trend was seen in rwTTNT from 2L initiation (overall: 19.6, Benda-CD20: 38.0, CHOP/CVP-CD20: 12.0, chemosalvage: 5.4, and other novel therapies: 19.6 months) and rwCRR for pts who received 2L (overall: 47.7%, Benda-CD20: 57.1%, CHOP/CVP-CD20: 34.2%, chemosalvage: 34.6%, and other novel therapies: 60.0%).
Conclusions: Among pts who experienced POD24 and received qualified therapies, 2L outcomes highlight continued unmet need despite recent improvements. Future research is required to identify this subset of high-risk pts at diagnosis and to investigate prospectively the effect of novel therapeutic approaches on their treatment outcomes.
Disclosures
Zettler:C OTA: Current Employment. Fernandes:COTA: Current Employment. Belli:COTA: Current Employment, Current equity holder in private company. Hansen:COTA: Current Employment, Current equity holder in private company. Wang:COTA: Current Employment, Current equity holder in private company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal